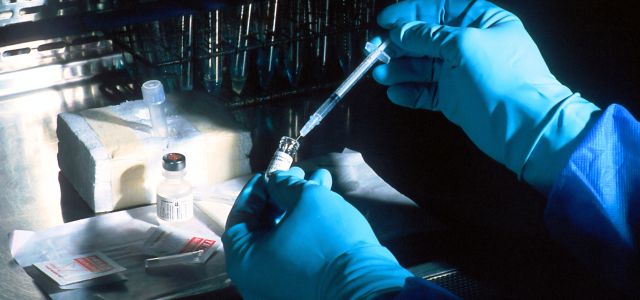
In an important decision that has significant implications for patent enforcement in the biotherapeutic and cell culture arts, the Federal Circuit has reaffirmed the International Trade Commission’s (ITC) authority to block the importation of infringing. The ruling in Wuhan Healthgen Biotechnology Corp. v. ITC No. 2023-1389 (Fed. Cir. Feb. 7, 2025) strengthens patent enforcement for biotech companies, particularly small firms, against foreign importers in two respects:
- Lowering the barrier to bringing an infringement action in the ITC against an infringing importer.
- Eliminating a key defense against an ITC infringement action for the importation of a biologic product such as engineered proteins.
The case centered on US Patent No. 10,618,951, owned by Ventria Bioscience, which covers recombinant human serum albumin (rHSA) produced in genetically modified plants for use in cell culture media. Ventria filed a complaint with the ITC alleging that Wuhan Healthgen (Healthgen) imported rHSA products infringed its patent. The ITC ruled in Ventria’s favor, finding both infringement and sufficient domestic industry. On appeal, Healthgen argued that Ventria had not satisfied the domestic industry requirement for bringing an ITC infringement action and that its products should be considered non-infringing upon arrival in the US due to post-manufacture changes and challenged whether Ventria had made enough US-based investments to satisfy the domestic industry requirement. The Federal Circuit affirmed the ITC’s findings.
Requirements for bringing an ITC infringement action
The ITC provides a powerful mechanism to block infringing imports through the use of an exclusion order. However, to bring an ITC action, per 19 U.S.C. § 1337 (often referred to as Section 337), the complainant must satisfy a domestic industry requirement, which consists of:
- Technical prong: at least one US product must practice the asserted patent.
- Economic prong: the patent holder must show significant investment in at least one of:
- Plant and equipment
- Employment of labor or capital
- Research, development, or licensing.
Historically, the economic prong posed challenges for small biotech firms and startups, which often invest heavily in R&D and licensing but not necessarily manufacturing, including plant and equipment, as they lack the capital and will often use a contract manufacturing organization (CMO) instead.
No fixed dollar threshold for Section 337 domestic industry requirement: a game changer for smaller firms
The Healthgen decision held that there is no rigid monetary threshold to satisfy the economic prong of the domestic industry requirement. The Federal Circuit upheld the ITC’s finding that Ventria Bioscience met the economic prong despite relatively modest investments because all of its rHSA-related R&D and production were US-based. The ruling makes clear that a small biotech startup with US-based research, process development, or regulatory compliance activities may still qualify under Section § 1337, even if it lacks a large manufacturing footprint. Additionally, the court noted that a company that conducts research and development domestically or licenses its technology can demonstrate a substantial investment in the exploitation of articles protected by the asserted patent, thus qualify under subsection (C) of § 1337(a)(3).
This is beneficial for early-stage biotech companies, whose core value lies in IP and R&D rather than large-scale production. By recognizing qualitative factors—such as the importance of domestic innovation efforts—the court’s decision helps small biotech firms compete more effectively against larger foreign manufacturers.
Rejection of the “post-manufacture alteration” defense
The Healthgen case strengthens patent enforcement for biotech companies by rejecting a potentially important loophole. Specifically, Healthgen argued that, while its rHSA products met the purity specifications covered by Ventria’s patent, they deviated from those parameters during transport and storage, making them non-infringing upon arrival in the US. The Federal Circuit dismissed this, finding Healthgen failed to show that such deviations changed the product enough and/or consistently to evade infringement.
This ruling is relevant for engineered proteins used for cell culture reagents and other applications, where stability, purity, and aggregation levels are key claim elements. Further, it affects temperature-sensitive biopharmaceuticals, such as antibodies, recombinant proteins, cell and gene therapies, and RNA-based treatments, which may degrade over time. Moreover, the decision impacts manufacturers relying on overseas production, as testing data from the manufacturing site can now be used as evidence of infringement, regardless of post-production alterations.
Strategic considerations for biotech patent practitioners and executives
For biotech patent attorneys and company executives, this ruling provides important guidance. First, small and mid-sized biotech firms can more easily satisfy the ITC’s domestic industry requirement. Companies with limited but US-focused R&D production should consider ITC enforcement, as they no longer need to prove large-scale capital investments—significance is now measured in context.
Second, patent claims should include measurable biochemical properties. The court’s focus on aggregation thresholds and stability data highlights how precise claim drafting can make a substantial difference in enforcement. To strengthen infringement arguments, claims should include quantitative metrics such as endotoxin levels, purity percentages, and degradation rates.
Third, ITC actions provide a faster and stronger enforcement mechanism than district court litigation. ITC cases typically conclude within 12-18 months, making them significantly faster than federal patent litigation. Further, the ITC’s exclusion orders, which block infringing imports, can be more valuable than monetary damages.
Conclusion
This ruling significantly lowers the barrier for small biotech companies to bring ITC enforcement actions, ensuring that innovators with US-based R&D and process development can effectively challenge foreign competitors. By confirming that there is no fixed dollar threshold for domestic industry, the decision expands ITC access to smaller firms, allowing them to leverage exclusion remedies against infringing imports.
Additionally, the court’s rejection of the post-manufacture alteration defense strengthens patent enforcement for biologically produced products (e.g., biologics), including engineered proteins, reinforcing that product properties at the time of manufacture—not post-importation changes—determine infringement liability.
For patent practitioners, the key lesson from the case is that biotech patent enforcement is evolving. The ITC is now a more accessible venue for small companies, and strategic claim drafting, investment structuring, and regulatory compliance activities will be critical for ensuring strong patent protection in the biologics marketplace.

Written by Joel M Harris
Senior Director, Intellectual Property, Atara Biotherapeutics, Inc.
You may also like…
Pravin Anand conferred with the APAA Enduring Impact Award
Pre-eminent IP Lawyer and Managing Partner of Anand and Anand, Mr Pravin Anand, has been conferred with the...
The quiet power of confidentiality clubs in SEP litigation
In standard essential patent (SEP) disputes, especially those involving FRAND (Fair, Reasonable, and...
A $10 million patent win reduced to a $1 lesson in damages
In a decision that will resonate as a stark warning to patent litigants, the US Court of Appeals for the Federal...
Contact us to write for out Newsletter